Xiaopei Li, Fan Yang, Yiran Li, Cheng Liu, Peng Zhao, Yi Cao and Huaping Xu, Yulan Chen
CCS Chem.; 2023, 5, 925.
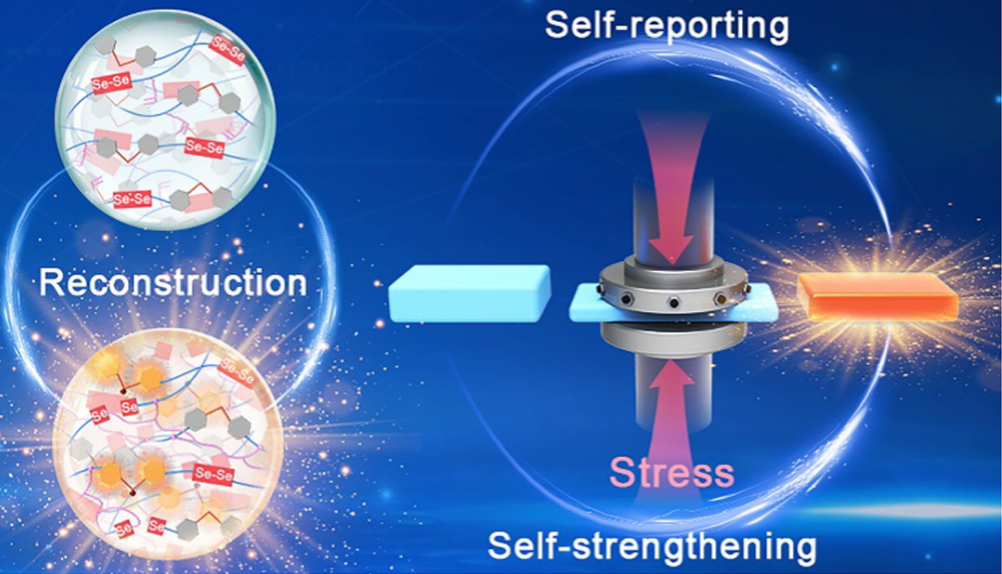
Unlike biological materials that can sense mechanical force and actively remodel locally, synthetic polymers typically break down under stress. Molecular-level responses to damage with both stress-reporting and self-strengthening functions are significant yet difficult to realize for synthetic polymers. To overcome this challenge, chemo-mechanical coupling into polymers that can simultaneously ameliorate mechanical, optical, or other functional properties of a polymer combined with mechanical treatment will offer a new principle for materials design. Here, we report a kind of elastomer in which destructive forces are channelled into productive and bond-forming reactions by using diselenide (Se–Se) as a mechanophore. Polyurethane has been functionalized with labile Se–Se bonds, whose mechanical activation generates seleno radicals that trigger radical transfer and cross-linking reactions in situ. These reactions are activated efficiently in a mechanical way by compression in bulk materials. The resulting covalent networks possess turn-on mechano-fluorescence and increased moduli, which provide the functions of stress reporting, mechano-healing, and mechano-remodeling for the deformed film. This study not only illuminates the mechano-responsive nature of Se–Se bonds in the bulk state but also paves the way for the development of new stress-responsive materials.