Jun Guan, Ruihao Zhou, Hengxue Shi, Shenghan Zhang, Chaowei He, Muqing Cao, Lei Jiao*, Huaping Xu*
Chem Catalysis; 2025, 5, 101429
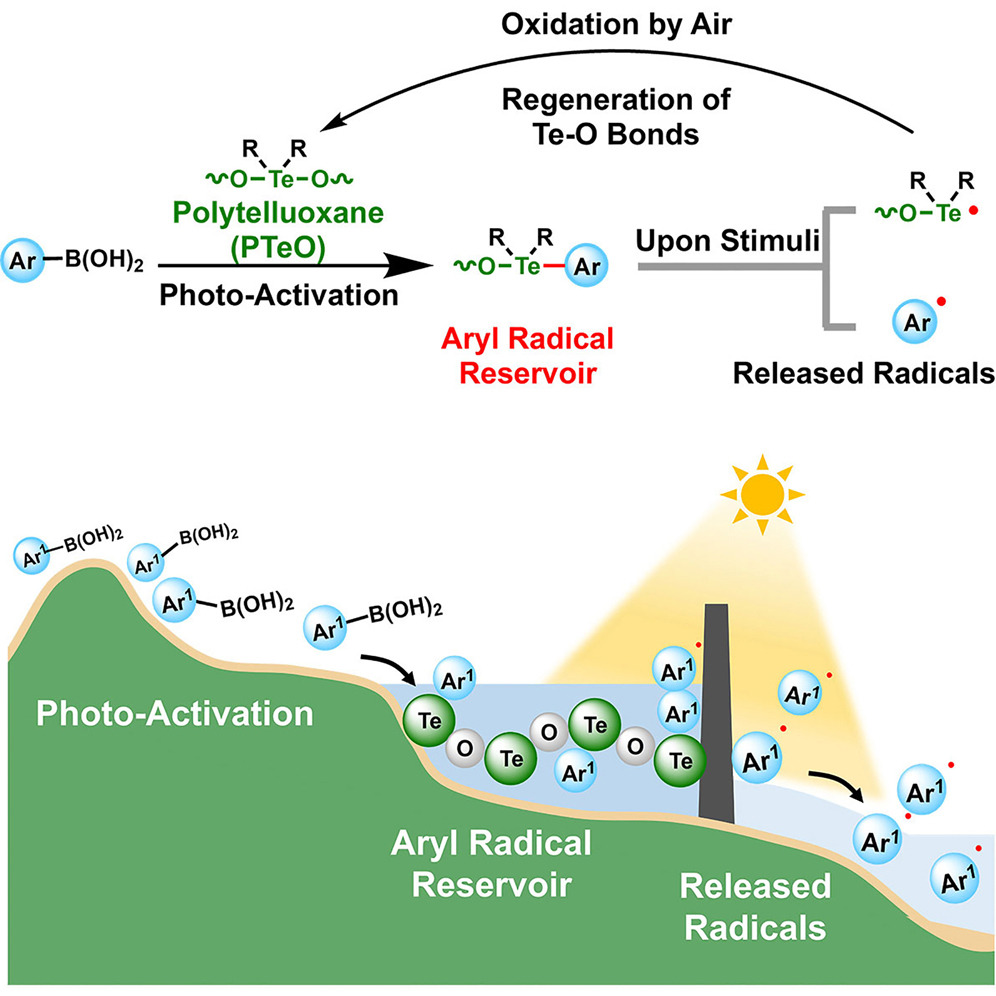
Carboradical reservoirs are non-radical precursors that controllably release stored radicals to directly participate in product formation, making them critically important in organic chemistry. However, no aryl radical reservoir has been reported to date. Here, we have constructed an aryl radical reservoir via polytelluroxane (PTeO)-mediated activation of arylboronic acids under white light. PTeO, featuring an inorganic Te–O backbone with organic side chains, facilitates the transfer of aryl substituents from boronic acids to Te sites, thereby storing them as reactive Te–C bonds and forming the reservoir. The stored radicals can be responsively released through the homolysis of Te–C bonds under white light or heating. Furthermore, air re-oxidizes the remaining Te radicals, restoring the Te–O backbone, regenerating PTeO, and imparting catalytic capability to PTeO. This work broadens the scope of synthetic methodologies and highlights the significant potential of PTeO in advancing organic synthesis.